
Mechasistic probe of isotopic labelling in the study of Favorskii mechanism: Synthesis of 2-chloro cyclohexanone starting from from Carbon-14.
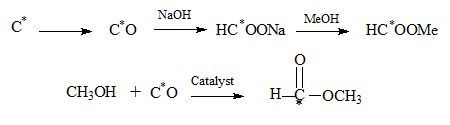
Starting from 14C several basic conversions are carried out leading to labelled methyl formate as shown. (C1 chemistry synthesis from 1 carbon reactants).The isotopic carbon by partial combustion is converted to CO which is absorbed in NaOH to result in methyl formate with the only carbon in it as an isotope. Alternatively the CO may be reacted with methanol in the presence of a catalyst to yeild the ester directly.
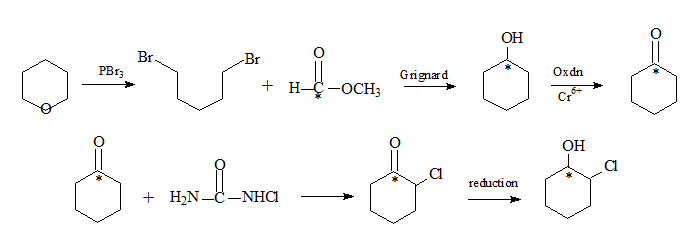
The synthesis of chlorocyclohexanone begins with tetrahydropyran (oxocyclohexane, IUPAC-oxane), condensed with 1,5-dibromopentane, the labelled methyl formate in presence of PBr3 followed by a Grignard reaction results in cyclohexanol with the carbon bonded to oxygen having the label. Chromium oxidation results in cyclohexanone. Reaction with chlorourea follwed by reduction yields chlorocyclohexanol with the label still in the same position. Chlorourea facilitates halogenation at the α-carbon through a free radical process.
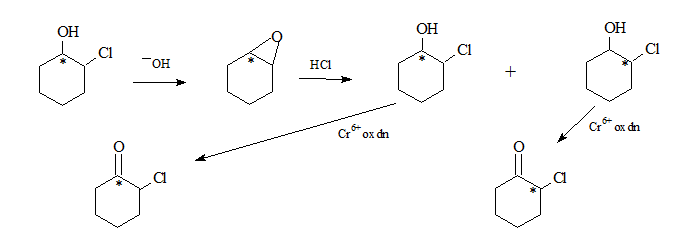
2-Chlorocyclohexanol in basic medium undergoes an internal SN2 to form the oxirane system.
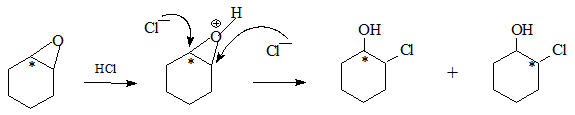
On acidification the oxirane opens up in two different ways with the label at the carbon bonded to Oxygen or the α-carbon as shown. Subsequent oxidation leads to the desired cyclohexanones.
Note: In aldehydes and ketones as in the cyclohexanone derivative the carbon next to the carbonyl group is refered to as the α-carbon (which has the halogen attached).
In chlorocyclohexanol, the numbering system is used thus, the carbon bonded to O is 1 and to chlorine is 2.